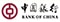100% security is the focus of PCBA in the medical field. Medical instruments and equipment require reliable, high-quality solutions.
POE provides fully integrated Class I, II and III medical device manufacturing capabilities. From production, assembly, packaging and logistics, POE brings OEM medical technology to life.
POE's biggest advantage in the field of medical PCBA is that it only manufactures products that fully comply with the latest standards of medical PCBA. We also have ISO 9001 and ISO 13485, two important certifications in the medical field.
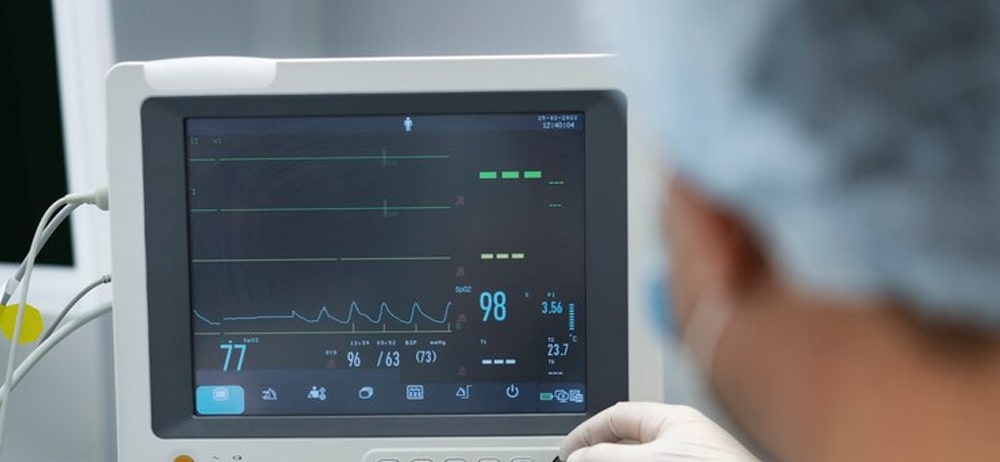
Our specialized technical capabilities meet the most stringent requirements of the medical industry, ensuring the highest quality outcomes for your projects.
Only produce medical PCBs with copper holes above 25um to ensure quality and reliability for medical devices.

Environmentally friendly lead-free PCB assembly services compliant with medical industry standards.

Rapid prototyping within 1 to 5 days, accelerating your medical device development process.
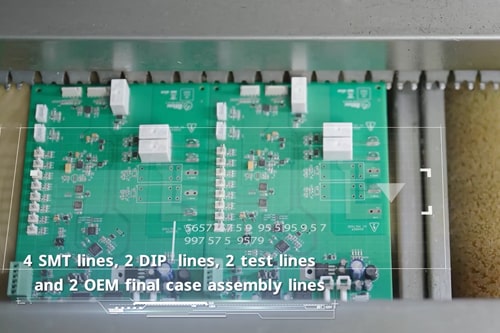
Rigid, flexible and up to 40-layer PCB assembly for complex medical equipment requirements.

Fine pitch, BGA and micro BGA mounting (74mm to 0.25mm) for high-precision medical devices.
Product specific functional testing ensuring reliability and performance of medical equipment.
Our commitment to quality is backed by internationally recognized certifications.

Quality Management System

Medical Devices QMS

Acceptability of Electronics

Flammability Standard
POE has rich PCBA experience in the following products in the medical field:
Power supplies, spectrum analysis, ultrasound, laser therapy, MRT and CT scans, respirators, infusion pumps, diagnostics, pacemakers, defibrillators, respirators, nursing monitors, electric wheelchairs, digital nutrition pumps, MRI equipment, patients Positioners, cochlear implants, scanning technology, control systems, insulin pumps, diagnostic equipment, cochlear implants, smartphones and tablets for mobile health applications, imaging equipment, 2D and 3D sensors, implantable defibrillators, magnetic resonance Imaging Equipment, Dental Equipment, X-ray Computed Tomography, Infusion Controls, Responsive Neurostimulators, Blood Glucose and Stress Monitors, Monitoring Equipment, Digital X-ray Equipment, Flexible and Rigid-Flex Interconnects for Sensitive Devices, Ultrasonic Equipment, Flow and distribution systems, body temperature monitors, imaging equipment, electrical muscle stimulation equipment, wearable medical devices, Ultra HDI circuit boards and cables.
"We have been working with POE for our medical device PCBAs for over 5 years. Their attention to detail and quality control processes are exceptional. Our products have consistently passed all regulatory requirements."
"The rapid prototyping service from POE helped us accelerate our product development cycle by 40%. Their technical expertise in medical PCBAs is unmatched in the industry."
"POE's clean room manufacturing environment and strict quality control measures give us confidence that our medical devices meet the highest standards. Their ISO 13485 certification was a key factor in our decision to partner with them."
Contact our medical PCBA experts today to discuss your requirements.
Reliability isn’t decided by the shell —
— POE PCB/PCBA Manufacturer (@poe_pcba) December 12, 2025
it’s the PCBA inside, built to stay stable under vibration, heat, and long operating hours.
That’s the part users never see,
but engineers and buyers know it’s where reliability is decided. #PCBA #HardwareDesign #Electronics #EMS pic.twitter.com/db7gyUhmUn

Since 1996, POE has become a globally renowned PCB company, providing small to medium volume PCB/ PCBA manufacturing services.